Applications
- Drinking water
- Iron removal processes and residual coagulant monitoring
- Industrial wastewater
- Measurement of effluents and wastewaters
- Boiler feed water
- Corrosion control
- Cooling water
- Surface water

Description
The 3S colorimeter is a compact analytical instrumentation for online analysis and process monitoring. The colorimeter is based on a color-developing chemical reaction carried out inside the analyzer's glass cell. The sample, coming from an external reservoir or process stream is autonomously grabbed by the instrument and mixed with the appropriate reagents to make the reaction happen. The analyzer uses an LED light source and a photodiode to photometrically measure the developed color intensity (absorbance). The absorbance is proportional to the concentration of the target chemical species (analite).
Features:
- Low reagents consumption and analytical reliability thanks to our custom designed miniature peristaltic pumps
- Dual compartment design to guarantee a complete separation between the electronics and the hydraulic section
- Color touchscreen to show the last measured value clearly, and to set all the options and settings via an user friendly interface
- Datalogger function with data history in graphical form and USB download
- Automatic calibration, validation and cleaning to reduce down time and operator intervention, their frequency can be freely set by the user
- Fully integrable in industrial automation via the analog outputs (2 x 4-20 mA channels), digital output (2 relays + ModbusRTU) and digital input (voltage free contact)
Iron is ubiquitous on Earh's crust, it can be found in varying amounts in surface waters. It can can also be found in potable water especially when used as coagulant in water purification processes.
The determination ranges of the iron analyzer vary from trace μg/L to 200 mg/L using the (optional) internal dilution module. Ranges also depend on the chemical method used.
Method
In an acid buffered solution, ferrozine and iron react to form a purple colored complex measured at 572 nm. The absorption intensity is proportional to the iron concentration in the sample.
Features
| Measured parameter | Fe2+/Fe3+ | ||||||
|
Range
|
|||||||
|
Reproducibility*
|
|||||||
| Limit of detection | 2 ppb | ||||||
| Wavelength | 572 nm | ||||
| Demineralized water | Not required | ||||
| Number of reagents | 1 | ||||
| Min. analysis time | 8 minutes | ||||
| Default analysis time | 15 minutes | ||||
|
Reagent consumption**
|
|||||
Reagents and standards
Safety Datasheet
In a slightly acidic buffered solution, 1,10-phenanthroline and ferrous ion react to form an orange color in proportion to the iron concentration. The Fe(II)-phenanthroline complex is quite stable and measured at 430 nm. The absorption intensity is proportional to the iron concentration in the sample. A reduction of Iron(III) to Iron (II) must first be carried out in order to measure both iron species.
Features
| Measured parameter | Fe2+/Fe3+ | ||||||
|
Range
|
|||||||
|
Reproducibility*
|
|||||||
| Limit of detection | 0.02 ppm | ||||||
| Wavelength | 430 nm | ||||
| Demineralized water | Not required | ||||
| Reference | ISO 6332:2009 | ||||
| Number of reagents | 2 | ||||
| Min. analysis time | 7 minutes | ||||
| Default analysis time | 20 minutes | ||||
|
Reagent consumption**
|
|||||
Reagents and standards
Safety Datasheet
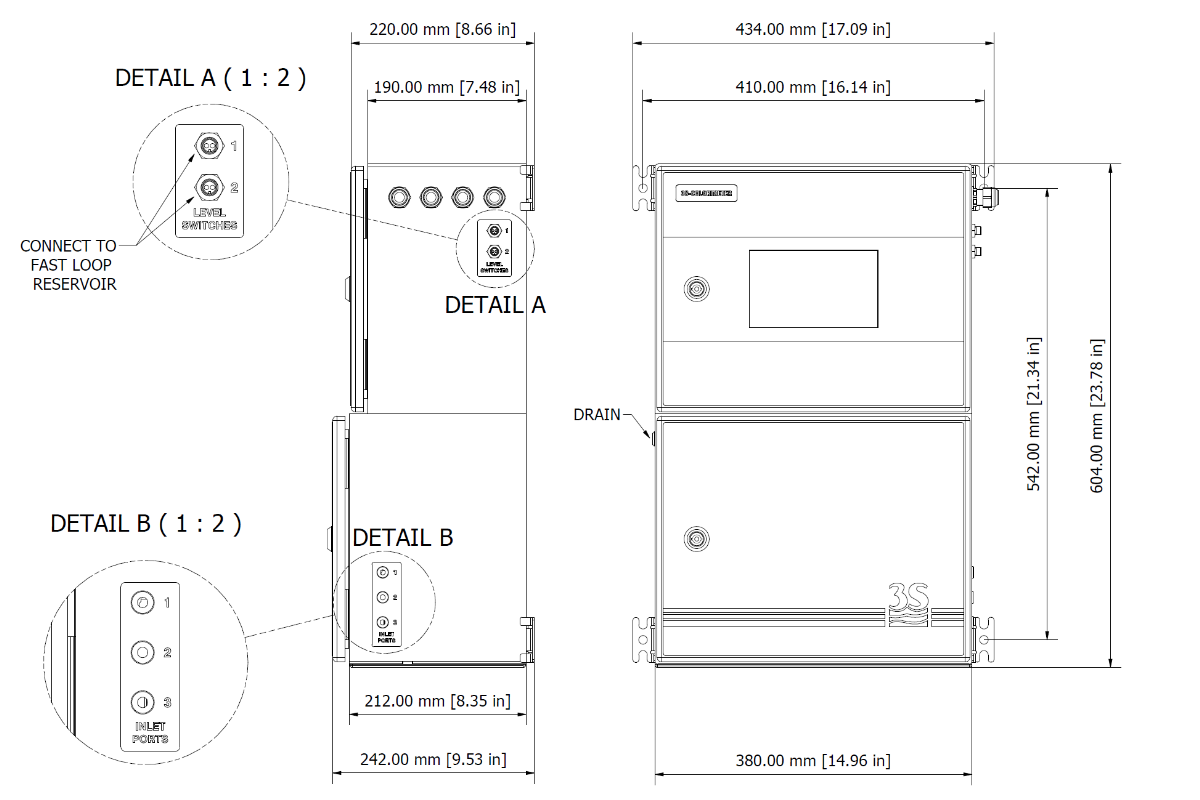
Technical Specifications
| Model | 3S-CL |
| Sampling Mode | Batch, with freely settable frequency. |
| Sample | Pressure: Atmospheric Temperature: 5 ‐ 45°C (41 ‐ 113°F) Flow Rate: 80 to 500 mL/min Connection: 6 mm (¼-in.) |
| Drain |
Pressure-free drain Connection: 12 mm (½-in.) |
| Input Streams | 2 |
| Material | Epoxy-coated stainless steel AISI 304 |
| Dimensions (H x W x D) | 604 x 380 x 210 mm (23.6 x 14.8 x 8.2 in) |
| Weight | 20 kg (44 lbs) |
| Power Supply |
Input Voltage: 115 VAC,230 VAC Power consumption: max. 80 W |
| Output signals |
2 x 4-20 mA analog outputs Modbus via RS485,Ethernet |
| Alarms | 2 SPDT programmable voltage-free relays |
| Digital Input | None, Online, Start Extra, Skip Idle, Emergency Stop |
| Ambient Temperature | 5 - 45 °C (41 - 113 °F) |
| Ambient Humidity | 10 - 90 % RH (non-condensing) |
| Protection Grade | IP54 (indoor only, outdoor use possible with external cabinet, not included) |
Documentation
Related Accessories
- SS external reservoir with level switch for dilution water
- Fast Loop external reservoir with level switch - polycarbonate
- SS External reservoir with level switch for sample
- PVC external reservoir with level switch
- ENCLOSURE FOR OUTDOOR OPERATION
- Filtration unit 100 micron 230 VAC
- Sampling Pump
- Stainless steel continuous flow D.50 sensor holder
- PVC continuous flow D.50 sensor holder
- STAINLESS STEEL CONTINUOS FLOW DOUBLE PROBE HOLDER
- Immersion pipe for online sensors